Research Scientific achievements and research interests
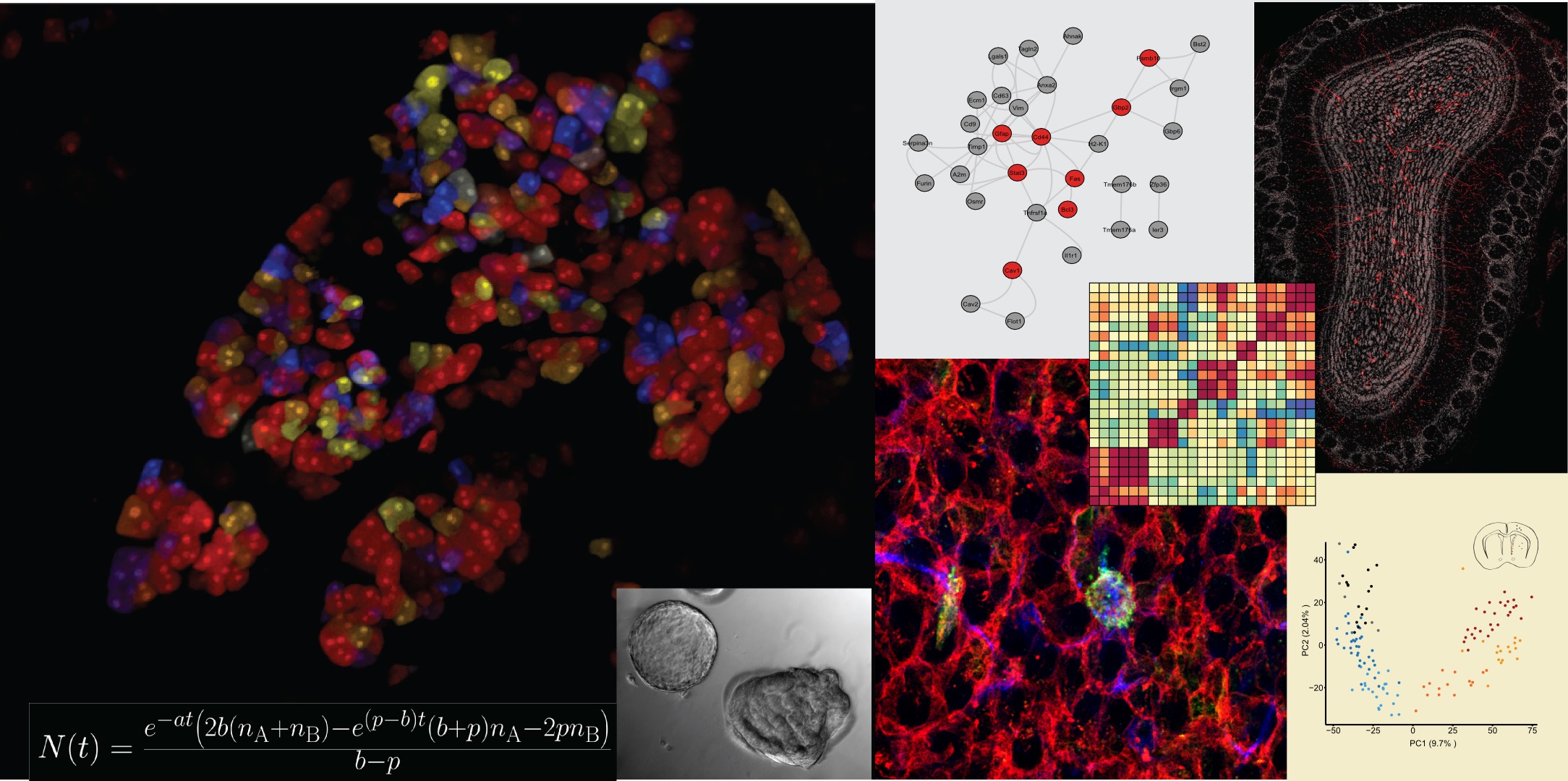
Left side: Tracing of acinar cells using Rainbow2-labeling in order to investigate homeostasis of adult pancreas, inset showing organoids resulting by proliferation of acinar cells in vitro under 3D culture conditions, formula describes the average number of nuclei in a clone, an important parameter for the mathematical modeling of acinar cell propagation.
Right side: Summary of recent data related to neural stem cell (NSC) biology, bottom left shows NSCs in the center of pinwheel structures (in green) in the subventricular zone of adult mice, single-cell sequencing demonstrated heterogeneity which is illustrated by principal component analysis (PCA) in the bottom right part. These NSCs are producing newborn neurons in the olfactory bulb throughout the life of a mouse, labeled with red fluorescent protein (RFP, see top right). Interferon signaling contributes to activation of quiescent NSCs when mice are subjected to brain injury as illustrated in the upstream regulator analysis on the top left.
Research Focus: Brain and Pancreas Regeneration
We concentrate our efforts on two vital organs known for their quiescence and their different regenerative capabilities:
1. Brain Regeneration
- Challenge: The brain exhibits minimal regenerative activity following injuries such as strokes.
- Our Approach: Investigate how astrocytes, the star-shaped glial cells in the brain, can be reprogrammed to gain facultative stemness, thereby enhancing the brain's innate ability to heal.
- Outcome: Develop strategies to improve regenerative responses in the aging brain, potentially mitigating the long-term impacts of neurological injuries.
2. Pancreatic Regeneration
- Challenge: While the pancreas shows a remarkable ability to fully regenerate after pancreatitis, the underlying mechanisms remain poorly understood.
- Our Approach: Study acinar cells in the pancreas to uncover how they acquire stemness and facilitate complete tissue regeneration.
- Outcome: Translate these insights to other organs with limited regenerative capacity, such as the brain.
Stem Cell Research: Unraveling the Secrets of Stemness
Understanding stem cells is pivotal to our mission of promoting regeneration. Our stem cell research encompasses several key areas:
- Single-Cell Technologies: We utilize cutting-edge single-cell imaging and sequencing technologies to dissect the molecular layers involved in the activation of stemness, the heterogeneity of stem cells, and their plasticity. This high-resolution approach allows us to pinpoint the exact mechanisms that govern stem cell behavior.
- Activation of Regeneration in the Aging Brain: Our goal is to activate regeneration in the aging brain by leveraging the plasticity of astrocytes. By understanding how to reprogram these cells, we aim to enhance the brain's natural healing processes.
- Facultative Stemness Acquisition: We study how injuries to the pancreas and brain lead to the acquisition of facultative stemness in acinar cells and astrocytes, respectively. This research helps us understand how certain cells can revert to a more stem-like state to facilitate tissue regeneration.
- Computational Analysis: We employ computational analysis of high-dimensional single-cell data to uncover patterns and insights that are not apparent through traditional methods. This involves sophisticated data processing and interpretation to advance our understanding of stem cell dynamics. This work is in collaboration with the Anders Group at Bioquant.
- Mathematical Modeling: Our team develops mathematical models to predict the dynamics of stem cell activation and regeneration. These models help identify the main parameters governing these processes, enabling us to manipulate and enhance regenerative outcomes effectively. This work is in collaboration with the Marciniak group at Mathematikom.
Cancer Research: Understanding Aberrant Regeneration
Cancer can be viewed as a byproduct of failed regenerative processes. Our cancer research focuses on the interplay between regeneration and tumor development:
- Aberrant Regeneration and Tumor Initiation: We investigate how regeneration may initiate tumors in the pancreas. By studying the faulty regenerative processes, we aim to identify the triggers that lead to uncontrolled cell growth and tumor formation.
- Regulation of Stem Cell Dynamics in Cancer: Our research examines how the parameters governing the dynamics of healthy stem cells are aberrantly regulated in cancer, specifically contributing to the tumor progression of glioblastoma. Understanding these aberrant regulations allows us to identify potential targets for therapeutic intervention.
- Mouse Models and Organoid Systems: Utilizing mouse models of stroke and pancreatitis alongside organoid models derived from both mouse and human tissues, we recreate the cellular environments necessary to study both regeneration and cancer development in controlled settings.
- Single-Cell Sequencing and Imaging: By pioneering single-cell sequencing and imaging technologies, we analyze the cellular and molecular changes that occur during both normal regeneration and cancer progression, providing a detailed landscape of cellular behavior.
Innovative Methodologies and Technologies
To achieve our research goals, we employ a suite of advanced methodologies:
- Mouse Models: Utilize models of stroke and pancreatitis to study in vivo regeneration and tumor initiation processes.
- Organoid Models: Develop both mouse and human tissue organoids to replicate and analyze organ-specific regenerative and cancerous mechanisms.
- Single-Cell Imaging and Sequencing: Pioneer cutting-edge single-cell technologies to dissect cellular behaviors and gene expression profiles at unprecedented resolution.
- Computational Analysis: Leverage sophisticated computational tools to analyze high-dimensional biological data, uncovering insights into cellular dynamics and interactions.
- Mathematical Modeling: Develop and apply mathematical models to predict biological processes and identify key regulatory parameters.
Interdisciplinary Collaboration: Where Biology Meets Computation and Mathematics
Our strength lies in our diverse team, which brings together experts from various fields:
- Biologists and Biomedical Scientists: Specializing in cell biology, biochemistry, pharmacology, and seeking clinical applications.
- Computational Biologists and Data Scientists: Developing sophisticated models and algorithms to interpret complex biological data.
- Mathematicians: Creating mathematical models to predict and understand the dynamics of regeneration and cancer initiation and progression.
- Clinicians: Bridging the gap between laboratory research and patient care to ensure translational impact.
By fostering a collaborative environment, we leverage the synergy of multiple disciplines to drive innovation and achieve our research objectives.
Major collaborators
Theodore Alexandrov, Spatial and single-cell metabolomics, EMBL Heidelberg, Germany.
Alexander Aulehla, Department of Developmental Biology, EMBL Heidelberg, Germany.
Ángel Carracedo Álvarez, Center for Research in Molecular Medicine and Chronic Diseases (CiMUS), Santiago de Compostella, Spain.
Simon Anders, ZMBH, Heidelberg, Germany.
Benedikt Berninger, KCL, London, UK.
Britta Brügger, BZH, University of Heidelberg, Germany.
Bernd Bukau, ZMBH, Heidelberg, Germany.
Helge Evers, Department of Biology, Chemistry, Pharmacy, Freie Universität Berlin, Germany.
Steve Goldman, Center for basic and translational Neuroscience, University of Copenhagen, Denmark.
Angela Goncalves, DKFZ, Heidelberg, Germany.
Dirk Grimm, Bioquant, Heidelberg, Germany.
Thomas Höfer, Division of Theoretical Systems Biology, DKFZ, Heidelberg, Germany.
Wolfgang Huber, Multi Omics and statistical computing, EMBL, Heidelberg, Germany.
Jeroen Krijsveld, Division of Proteomics of Stem Cells and Cancer, DKFZ Heidelberg, Germany.
Anna Marciniak, Inst. of Applied Mathematics, University of Heidelberg, Germany.
Raúl Méndez, IRB Barcelona, Spain.
Christoph Niehrs, DKFZ Heidelberg and IMB Mainz, Germany.
Christoph Plass, Divison Epigenomics, DKFZ, Heidelberg, Germany.
Carsten Schultz, Cell Biology and Biophysics, EMBL, Heidelberg, Germany.
Motomu Tanaka, Dept. Physical Chemistry of Biosystems, University Heidelberg, Germany.
Aurelio Teleman, DKFZ, Heidelberg, Germany.
Ilpo Vattulainen, Department of Physics, University of Helsinki, Finland.
Christian Wirtz, Dept. of Neurosurgery, University Hospital Ulm, Germany.
Funding
DFG SFB 1324 Mechniasm of Wnt/Hippo/CD95 signalosome in the orchestration of stem like and EMT phenotypes
DFG TRR 186 Spatio-temporal control of CD95-activation mode
ERC CoG ReBuild_CNS- Redirecting glila progenitor fate to rebuild the injured brain
DFG GRK 2727 FIne-tuning innate immunity for direct efficient repair of the diseased CNS
References
Corsini, N.S., Sancho-Martinez, I., Laudenklos, S., Glagow, D., Kumar, S., Letellier, E., Koch, P., Teodorczyk, M., Kleber, S., Klussmann, S., et al. (2009). The death receptor CD95 activates adult neural stem cells for working memory formation and brain repair. Cell Stem Cell 5, 178-190.
Drachsler, M., Kleber, S., Mateos, A., Volk, K., Mohr, N., Chen, S., Cirovic, B., Tuttenberg, J., Gieffers, C., Sykora, J., et al. (2016). CD95 maintains stem cell-like and non-classical EMT programs in primary human glioblastoma cells. Cell Death Dis 7, e2209.
Gao, L., Brenner, D., Llorens-Bobadilla, E., Saiz-Castro, G., Frank, T., Wieghofer, P., Hill, O., Thiemann, M., Karray, S., Prinz, M., et al. (2015). Infiltration of circulating myeloid cells through CD95L contributes to neurodegeneration in mice. JExpMed 212, 469-480.
Kleber, S., Sancho-Martinez, I., Wiestler, B., Beisel, A., Gieffers, C., Hill, O., Thiemann, M., Mueller, W., Sykora, J., Kuhn, A., et al. (2008). Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell 13, 235-248.
Letellier, E., Kumar, S., Sancho-Martinez, I., Krauth, S., Funke-Kaiser, A., Laudenklos, S., Konecki, K., Klussmann, S., Corsini, N.S., Kleber, S., et al. (2010). CD95-ligand on peripheral myeloid cells activates Syk kinase to trigger their recruitment to the inflammatory site. Immunity 32, 240-252.
Llorens-Bobadilla, E., Zhao, S., Baser, A., Saiz-Castro, G., Zwadlo, K., and Martin-Villalba, A. (2015). Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells that Become Activated upon Brain Injury. Cell Stem Cell 17, 329-340.
Martin-Villalba, A., Llorens-Bobadilla, E., and Wollny, D. (2013). CD95 in cancer: tool or target? Trends MolMed.
Sancho-Martinez, I., and Martin-Villalba, A. (2009). Tyrosine phosphorylation and CD95: a FAScinating switch. Cell Cycle 8, 838-842.
Seib, D.R., Corsini, N.S., Ellwanger, K., Plaas, C., Mateos, A., Pitzer, C., Niehrs, C., Celikel, T., and Martin-Villalba, A. (2013). Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell 12, 204-214.
Teodorczyk, M., Kleber, S., Wollny, D., Sefrin, J.P., Aykut, B., Mateos, A., Herhaus, P., Sancho-Martinez, I., Hill, O., Gieffers, C., et al. (2015). CD95 promotes metastatic spread via Sck in pancreatic ductal adenocarcinoma. Cell DeathDiffer.
Teodorczyk, M., and Martin-Villalba, A. (2010). Sensing invasion: cell surface receptors driving spreading of glioblastoma. JCell Physiol 222, 1-10.
Wollny, D., Zhao, S., Everlien, I., Lun, X., Brunken, J., Brüne, D., Ziebell, F., Tabansky, I., Weichert, W., Marciniak-Czochra, A., et al. (2016). Single-Cell Analysis Uncovers Clonal Acinar Cell Heterogeneity in the Adult Pancreas. Developmental Cell.
Ziebell, F., Martin-Villalba, A., and Marciniak-Czochra, A. (2014). Mathematical modelling of adult hippocampal neurogenesis: effects of altered stem cell dynamics on cell counts and bromodeoxyuridine-labelled cells. JRSocInterface 11, 20140144.
Zuliani, C., Kleber, S., Klussmann, S., Wenger, T., Kenzelmann, M., Schreglmann, N., Martinez, A., del Rio, J.A., Soriano, E., Vodrazka, P., et al. (2006). Control of neuronal branching by the death receptor CD95 (Fas/Apo-1). Cell DeathDiffer 13, 31-40.